
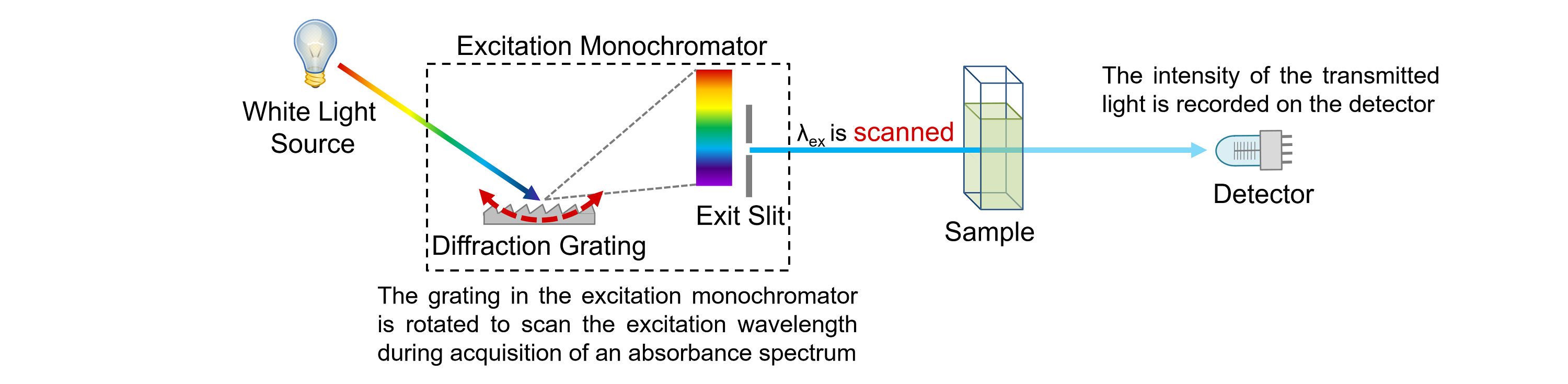
The emission spectra of atoms in the gas phase, on the other hand, do not show a continuous spread of wavelength from red to violet, rather they emit light only at specific wavelengths with dark spaces between them. The spectrum of the visible light, is continuous as all wavelengths (red to violet) of the visible light are represented in the spectra. The study of emission or absorption spectra is referred as spectroscopy Line Spectrum The wavelengths which are absorbed are missing and come as dark lines.Īn absorption spectrum is like the photographic negative of an emission spectrum Spectroscopy The sample absorbs radiation of certain wavelengths. Absorption SpectrumĪbsorption spectrum is the spectrum obtained when radiation is passed through a sample of material. Sample gives up the absorbed energy, is recorded. It is noticed when radiations emitted from source are passed through a prism & received on photographic plate.Įmission spectrum is produced by supplying energy to a sample by heating it or irradiating it and the wavelength (or frequency) of the radiation emitted, as the The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum. Hence, this spectrum known as continuous spectrum. These colors are so continuous that each of them merges into the next. When white light is analyzed by passing through prism, it splits into seven colors.
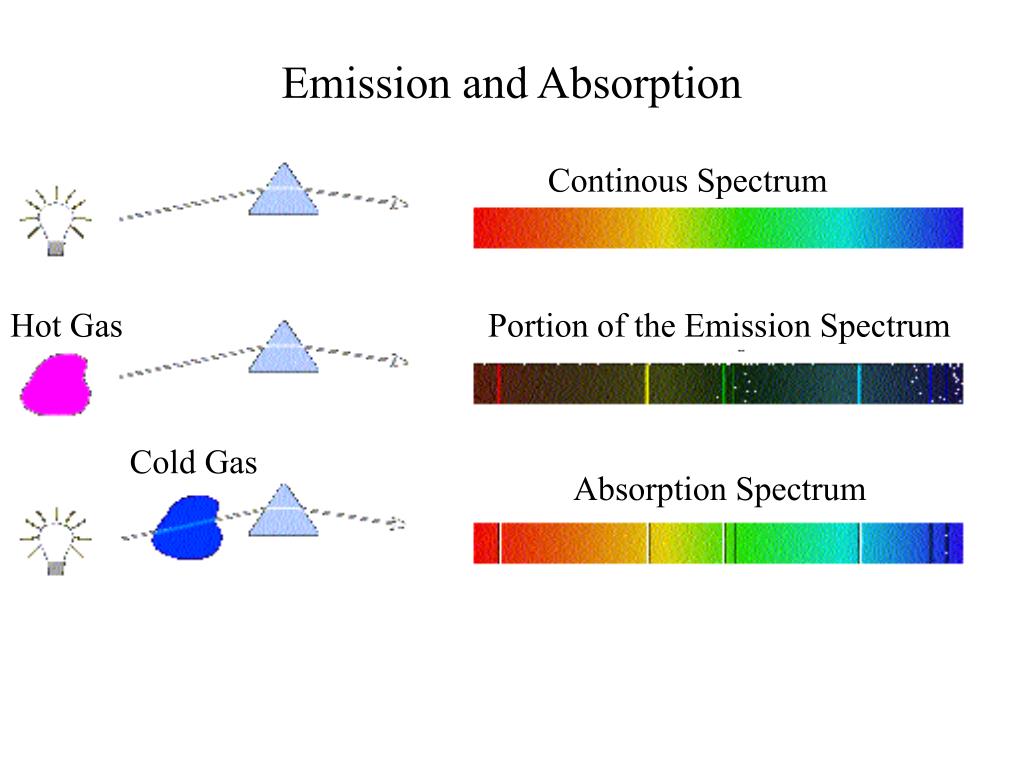
VIBGYOR (V) = Shortest wavelength 400nm R = Longest wavelength 450 nm Continuous spectrum See What are the various physical mechanisms for energy transfer to the photon during blackbody emission? for information about other mechanisms.When white light is passed through prism, it splits into band of seven colors called spectrum.

H $_2$ has a molecular bond, which has vibrational and rotational states. If you look in other directions, you see light that was headed straight away from the sun, but has now been scattered in another direction.Ģ} Thermal radiation from room temperature hydrogen isn't just caused by promoting electrons to higher energy orbitals. So if you look directly at the sun, it is less blue than it would appear in space. More blue is scattered than other colors. As light from the sun travels through the atmosphere, some of it is scattered in all directions from small particles. The sky is blue for a somewhat related reason. If you look in other directions you will see the emitted light. So if you look at the light shining through the gas, you see the missing light as dark lines in the spectrum. It will then return to the ground state and emit that light in all directions. Hydrogen will absorb specific wavelengths from that light and get promoted to an excited state. Please explain it using simple language, which don't involve so many complicated terms because I am at high school level.ġ} For the absorption spectrum, you have to shine a light through the gas. I am a high school student and I am very confused in absorption and emission spectrum of gases, for e,g take hydrogen at room temperature for simplicity, so that we can talk in terms of Bohr's model of atomġ)We know that when the white light is passes through it, some of the wavelengths will get absorbed by it and electron will be excited to higher energy level, but we also say that when it will return back it will emit he photon of same wavelength, so my question is would these both happens just one after another? i.e will the electron return just after it? but if that's the case it would re-emit is simultaneously so we shouldn't see the dark lines which we usually see?Ģ}we also know that the any body at any temperature also radiates and if it is at room temperature most of the radiation will be in infrared region, but in this case we are observing that hydrogen will give the photon of lower wavelengths also like when electron would return back from 1st excited state to ground level it will emit radiation of about 122.5nm which lies in visible range, so how is that possible? it is not in accordance with what we see that all bodies at room temperature mostly radiates in infrared region?


 0 kommentar(er)
0 kommentar(er)
